Non-polar molecule: symmetrical and at least one dipolar bond. (value of the difference is less than 0.5) These would be covalent bonds. Thus, the electrons are shared equally.
here are more examples!
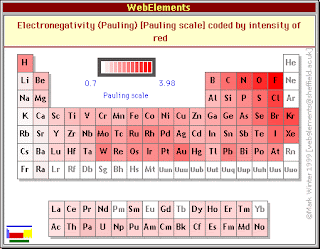
Positive ions, also known as electropositive, have very low electronegativities. Here is an electronegativities table to help you out! As you go up the column, the electronegativity increases.



No comments:
Post a Comment