As we are in a chemistry class, its important to be aware of all the harzards. WHMIS, or Workplace Hazardous Materials Information System is an international system that educates people about the dangers of the chemicals they are working with.
MSDS, or Material Safety Data Sheets are labels that can be found on all the chemical containers we will be working with this year. Here are some of the thing they can tell you:
WHMIS Symbols
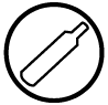 Compressed Gas
Compressed Gas- gas under pressure
- heat or having the container dropped may cause it to explode
- the gas isn't toxic unless there is another WHMIS symbol which will tell you the hazards
Flammable and Combustable
- can burn at a low temperature- even sparks can cause it to ignite
- several divisions: flammable gases, flammable liquids, combustible liquids, flammable solids, flammable aerosols, and flammable reactive materials(can ignite either in air or water)
- KEEP AWAY FROM HEAT!
 Oxidizing Materials
Oxidizing Materials-they release oxygen, or another chemical which will result in a similar effect, that helps fuel the fires of other flammable or combustible objects
- fire and explosion risk
- NEVER SMOKE NEAR CHEMICALS and wear proper protection(lab coats, goggles, etc.)
 Poisonous and Infectious
Poisonous and Infectious- contact with chemicals have immediate and serious effects
- can easily kill you
- can be classified as very toxic or toxic
- either way, wear appropriete clothes on a lab day and be sure to wash your hands afterward
 -Materials causing other Toxic Effects
-Materials causing other Toxic Effects- although they are poisonous, they don't have immediate effects
- exposure to the chemicals over long periods of time may leave permanent damage(cancer, birth effects, etc.)
- work in well ventilated areas
-Biohazardous Infectious Material
-organisms like bacteria and viruses which produce toxins which are thought to be responsible to diseases like HIV
Corrosive Material
- caustic or acidic chemicals which corrode (aluminium, flesh)
- can be gases or liquids(ammonia, sodium hydroxide)
Dangerously Reactive Materials
- things like heat, presure, etc. cause some materials to have dangerous reactions(e.g. decomposition)
International Safety Symbols
Not everyone uses WHMIS symbols. The International Safety Symbols have similar pictures, but the amount of sides the picture is surrounded by determines how dangerous the material is. They come in octagons, diamonds and triangles. The more sides, the more dangerous.
Safety in the Science Classroom
Much of safety is common sense. However, to prevent a situation like Spongebob's experiment, let's review. Here is a basic list of things to remember.
Always read the lab instructions before you start.
Clear your area of papers, books, and clutter.
Tie back long hair and wear closed toed shoes. Wear your goggles over your eyes. Lab coats must be done up.
Never eat in a science classroom.
Never leave your station unattended(Bunsen burners, open chemicals, etc.)
Never use unlabelled chemicals.
Check for chipped materials before you start the lab.
Don't run.
Never put used chemicals into the bottle from which they came. They could be contaminated.
Never pour them down the drain either unless the teacher says so.
Know where the first aid equipment and other emergency things are located(fire blanket, safety shower, fire alarm, emergency exit, phone, eye-wash station, first aid kit, etc) in your classroom.
Wash and dry your work area and all the materials you used. Put them back exactly where you got them from.
Put broken glass in the broken glass bin.
Add acid to water, not the other way around.
Be aware of hot plates and Bunsen burners. Never reach across open flames. Hot plates may not appear hot, but they stay hot for up to an hour and will burn you.
Don't let anything boil until it has dried up.
Waft vapours toward you, don't directly sniff the substance.
If you are burned, or have splashed chemicals onto your skin or eyes, run them under cold water for 15 mins and then tell your teacher.
Apply pressure to cuts to stop bleeding.
Identify the chemical.
If you get a job, make sure you know the hazards of your position. Teens have the highest rate of being injured on the job in BC.
_________________________________________________________________________
Now that you have had a review, draw in the background of the following picture with things that you shouldn't do during a lab.