In Lab 3B, one was able to experiment on Chromatography (particularly on sheet chromatography)
Sheet chromatography is a separation technique that involves using a thin layer of absorbent sheet. In the lab, we dotted a spot on each strip of sheet and dipped it into water. Slowly, the colours spreaded and we were able to see the components that made up each colour as it develops. With the experiment, we were able to calculate the Rfs (ratio of fronts) of each colour. We could calculate the Rf by using this formula: Distance #1 divided by Distance #2. In other words. the distance from D1 (where the spot spreaded to) to origin divided by solvent front (where the water rose to) to origin.
*the Rf value will always be between 0 to 1.
With the Rf determined, we were able to distinguish the component colours. (secondary colours separated into primary colours)
Overall, the lab was a very interesting experience.
Welcome...
This page belongs to a small goup of stundents who put in time for blog posts regarding the gr.11 chemistry curiculum. We post blogs of previous class lessons in our own terms for future use and for other students who find use of it. Enjoy... :D
Search This Blog
Thursday, October 20, 2011
Naming Ionic and Covalent Compounds
Ionic Compounds
- made of negatively and positively charged particles(metal and a non-metal)
- to try and make themselves stable, atoms transfer electrons and become ions
- take the valence shell configuration of the nearest noble gas
- make ionic lattices in solids

To write its name, basically reverse the steps you did to write its symbol. However, before you start, there are several things you should know. Unless you're naming compounds with polyatomic ions, change the ending of the anion to "ide". To write multivalent metals you must include the charge in brackets in roman numerals.
Let's work through several examples:
ZnF2 = Zn+2 and F- ->Zinc Fluoride
AuCl3 = Au+3 and Cl- ->Gold (III) Chloride
Fe2(SO3)3 = Fe+3 and SO3-2 -> Iron(III) Sulphite
If you want to get some practise, here's a great site:
http://www.teacherbridge.org/public/bhs/teachers/Dana/ionic.html
Covalent Compounds
- two non-metals
- share electrons and become a molecule
- valence shells take the formation of the nearest noble gas
-diatomic molecules exist in our environment: H2, N2, O2, F2, Cl2, I2, and Br2.
Naming covalent compounds is much easier than ionic compounds. Similar to ionic compounds, cross the charges. All you have to do is add a prefix depending on the amount of particles there are.

Let's work through some examples:
Carbon dioxide = C and since the prefix is "di", there are 2 oxides. -> CO2
Carbon tetrafluoride = CF4
N2 = nitrogen gas
SF6 = Sulphur hexafluoride (only add "mono" if it's only one on the second element)
Si2Se4 =disilicon tetreselenide
Cl2Se = Dichloride monoselenide
- made of negatively and positively charged particles(metal and a non-metal)
- to try and make themselves stable, atoms transfer electrons and become ions
- take the valence shell configuration of the nearest noble gas
- make ionic lattices in solids
To write ionic compounds, you need a metal and a non-metal. For example, let's work with Lithium Fluoride. The first element in a name is always the metal ion. Find the two components and their charges. In this case its Lithium+ and Fluorine-. Switch the charges on the atoms and write the compound out. The balanced charges should be written on the lower right corner of the symbol. LiF(in this case since the charges are both 1, you don't need to write them)
Let's work through a few more:
Magnesium Chloride = Mg+2 and Cl-1 -> Mg1 and Cl2 -> MgCl2
Sodium Sulphide = Na+ and S-2 -> Na2 and Cl1 -> Na2Cl
Beryllium oxide = Be+2 and O-2 -> Be2 and O2(cancel each other out!) -> BeO
When working with polyatomic ions, refer to the chart. Put the whole ion in brackets if charges cross.
Potassium Chlorate = K+ and ClO3- -> KClO3
Chromium (II) Nitrate = Cr+2 and NO3- ->Cr(NO3)2
Common Polyatomic Ions

Let's work through several examples:
ZnF2 = Zn+2 and F- ->Zinc Fluoride
AuCl3 = Au+3 and Cl- ->Gold (III) Chloride
Fe2(SO3)3 = Fe+3 and SO3-2 -> Iron(III) Sulphite
If you want to get some practise, here's a great site:
http://www.teacherbridge.org/public/bhs/teachers/Dana/ionic.html
Covalent Compounds
- two non-metals
- share electrons and become a molecule
- valence shells take the formation of the nearest noble gas
-diatomic molecules exist in our environment: H2, N2, O2, F2, Cl2, I2, and Br2.
Naming covalent compounds is much easier than ionic compounds. Similar to ionic compounds, cross the charges. All you have to do is add a prefix depending on the amount of particles there are.

Let's work through some examples:
Carbon dioxide = C and since the prefix is "di", there are 2 oxides. -> CO2
Carbon tetrafluoride = CF4
N2 = nitrogen gas
SF6 = Sulphur hexafluoride (only add "mono" if it's only one on the second element)
Si2Se4 =disilicon tetreselenide
Cl2Se = Dichloride monoselenide
Tuesday, October 18, 2011
Separation
Separation techniques:
The components in a mixture retain identity throughout each different technique, and the more similar the components are the more difficult it is to separate the substance. The strategy is to create a process that makes the identities of properties in the component clear.
The Techniques:
- Filtration: Substances go through what is called a Porous Filter, or something simpler like filter paper, either makes the residue stay which the solid does not dissolve, and the filtrate goes through the paper.
- Floatation
- Hand Separation: mixtures separate by a magnet or sieve, usually a mechanical mixture or heterogeneous mixture.
- Evaporation: Simply the substance is boiled so that the water evaporates and the liquid substance is left.
- Crystallization: Like solid to liquid, precipitation is created as solids separating by filtration or floatation, this becomes the saturated solution of a solid. After the evaporation or cooling the substance comes out as a pure crystal (Crystal Filtered).
- Gravity Separation: ( Solids based on density) Substances are placed in a test tube and is whirled around in a centrifuge at high speeds, the denser materials go to the bottom. Gravity Separation works best with small amounts.
- Solvent Extraction: The Mechanical Mixture: Use liquid to dissolve one solid but not the other, so that the desired solid is left behind or dissolved. Solution: The solvent is soluble with the solvent already present.
- Distillation: Heating a mixture at a low-boiling point so that the components are able to volatilize. Its the process that collects and condenses flow over different speeds
- Chromatography: (Meaning colour and writing in Greek) The flow of a mixture over a material (different components flow over different speeds).-Mobile Phase: Sweep the sample over a stationary phase-Stationary Phase: A liquid soaked into a sheet or a strip of paperPaper Chromatography (PC): Liquid soaked up into a sheet of paper which is a stationary phase. After observation, the components appear separated (the developing spot spreads).Thin Layer Chromatography (TLC): A layer of an absorbent coating a sheet of plastic or glass, the components bond strongly or some bond weakly (Al²O³ or SiO², usually used).
A Short Clip of the Student Lab in Class: Paper Chromatography
Sunday, October 16, 2011
Chemical Change vs. Physical Change: Can YOU Spot the Difference?

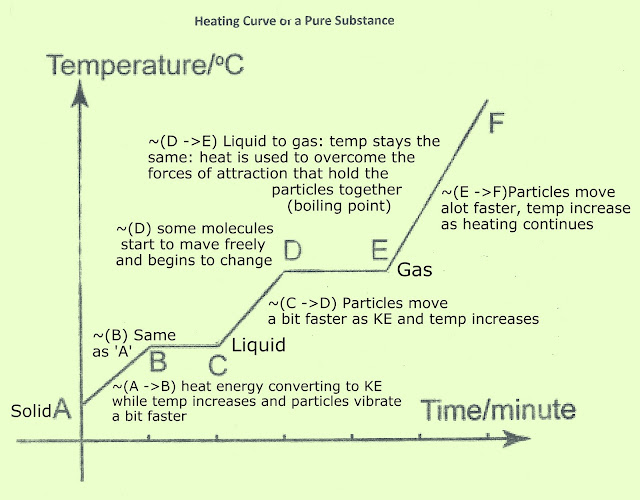
We began learning about chemical and physical changes in class. We learnt that a chemical change occurs when when a new substance is made, & old bonds are broken while new ones are made. An example of a chemical change would be cooking an egg On the contrary, physical changes DO NOT produce a new substance. An example would be ice melting --> the ice melts into water however the same substance is present, there's just a change in state.
The diagram below shows the changes in state when they undergo any physical changes :
- colour change
- change in state
- odour
- light
- heat
Ex. H2O (s)à H2O (l)
I noticed that the reactant was the same as the product, the only difference is the change in state from a solid (s) to a liguid (l).
Saturday, October 15, 2011
Naming Acids
Acids that have only 2 components are called binary acids. Usually they contain hydrogen and another element. E.g. HF- hydrofluoric acid.
To name them, put the prefix 'hydro' infront of the anion and change the the ending to 'ic'.
E.g. -HCl -> hydro + (choride = chloric) + acid -> hydrochloric acid
-HBr -> hydro + (bromide = bromic) + acid -> hydrobromic acid
-HCN -> hydro + (cyanide = cyanic) + acid -> hydrocyanic acid
Naming Complex Acids
Complex acids are acids which have hydrogen and a polyatomic anion.
 To name them, first find the anion. If the anion ends in 'ate', change the ending to 'ic', but if the anion ends in 'ite', change the ending to 'ous'. Add 'acid' to the end.
To name them, first find the anion. If the anion ends in 'ate', change the ending to 'ic', but if the anion ends in 'ite', change the ending to 'ous'. Add 'acid' to the end.E.g. -HClO4 -> (perchlorate = perchloric) + acid -> perchloric acid
-H3PO4 -> (phosphate = phosphic) + acid -> phosphic acid
-HNO2 -> (nitrite = nitrous) + acid -> nitrous acid
For more information, visit:
Sunday, October 2, 2011
MATTER !!!
Matter is a substance that has MASS and takes up VOLUME. Matter has 3 states: solid, liquid, gas.
There are two types of matter: pure substances and mixtures.
Pure substances:
- fixed composition
- contains atoms of only 1 kind
-fixed physical and chemical properties
-fixed ratios of elements
Pure substances are divided into two more subgroups: elements and compounds
Elements:
-cannot be broken down to further substances
- examples: Helium, Carbon, and Aragon.
-made out of atoms: metals, metalloids, non-metals
Compounds:
-formed by two or more elements.
-example: water (2 hydrogen + 1 oxygen)
-examples: Methane, carbon dioxide, and sodium chloride.
-can be either covalent (organic compound) or ionic (acid, salt, base).
Mixtures:
-contain at least two substances
- not chemically combined
-can be separated into pure compounds or elements
-has a changing set of physical properties
There are two types of mixtures: Homogeneous and Heterogeneous.
Homogeneous:
-"homo" --> same
-has uniform properties and compositions throughout.
-commonly referred to as solutions
-example: table salt mixed in water
*it can be either a solution or a colloid*
Heterogeneous:
-"hetero" --> difference
-containing two or more substances, but not "mixed".
-contains different visible substances seen with the naked eye.
-example: cake mix and cookie dough
dried beans, rice, and vegetables mixed together is an example of a heterogeneous mixture
*heterogeneous can be either suspension or mechanical mixture.*
There are two types of matter: pure substances and mixtures.
Pure substances:
- fixed composition
- contains atoms of only 1 kind
-fixed physical and chemical properties
-fixed ratios of elements
Pure substances are divided into two more subgroups: elements and compounds
Elements:
-cannot be broken down to further substances
- examples: Helium, Carbon, and Aragon.
-made out of atoms: metals, metalloids, non-metals
Compounds:
-formed by two or more elements.
-example: water (2 hydrogen + 1 oxygen)
-examples: Methane, carbon dioxide, and sodium chloride.
-can be either covalent (organic compound) or ionic (acid, salt, base).
Mixtures:
-contain at least two substances
- not chemically combined
-can be separated into pure compounds or elements
-has a changing set of physical properties
There are two types of mixtures: Homogeneous and Heterogeneous.
Homogeneous:
-"homo" --> same
-has uniform properties and compositions throughout.
-commonly referred to as solutions
-example: table salt mixed in water
Table salt mixed into water creates a homogeneous mixture. One is unable to see the different substances when mixed together.
*it can be either a solution or a colloid*
Heterogeneous:
-"hetero" --> difference
-containing two or more substances, but not "mixed".
-contains different visible substances seen with the naked eye.
-example: cake mix and cookie dough
dried beans, rice, and vegetables mixed together is an example of a heterogeneous mixture
*heterogeneous can be either suspension or mechanical mixture.*
DIAGRAM OF MATTER
Saturday, October 1, 2011
Chemical and Physical Changes
Solids
-have a set mass and volume
-particles are arranged in regular patterns
-particles can only vibrate in place
Liquids
-take the shape of their container
-enough room between particles to slide past one another
Gases
-move freely
-undefined mass and volume

-have a set mass and volume
-particles are arranged in regular patterns
-particles can only vibrate in place
Liquids
-take the shape of their container
-enough room between particles to slide past one another
Gases
-move freely
-undefined mass and volume

Subscribe to:
Posts (Atom)