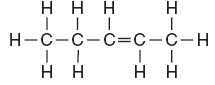
- general formula = CNH2N
- more reactive as opposed to straight-chains
- numbering can start anywhere on the ring, but the #'s of the side chains must be the lowest possible numbers
- prefix "cyclo-" is added to the beginning of the parent chain
Naming
Cycloalkanes
- count length of carbon chain
- similar naming rules as straight-chains
- if the numbers of the side groups are equal (ex. 2,4,6 either way you count) then you give the lower number to the side group that comes first in the alphabet
Cycloalkenes & Cycloalkynes
- similar naming to cycloalkanes, except with different endings
- more reactive than cycloalkanes
- when numbering the double/triple bonds, they should always be located at the first and second carbons
Aromatics
Properties
- electrons are "delocalized" meaning they can move around the ring and are shared equally in the ring
- carbon-carbon bonds have same reactivity because of delocalization
- less reactive structures than cycloalkanes
- have a nice odour
- contains atleast one benzene ring
- can be a parent or side chain
- as a side chain, its given the name "phenyl"
- as a parent chain, "benzene" is added to the end of the whole name
Ex. Name the structures:
cyclopentyne
4-chlorocyclohexene
All this organic chemistry is tough! It's a lot to memorize and there are a lot of rules to follow. But rest assured, it gets easier. Try these exercises. After all, practice makes perfect right? :)