We went to the computer lab to do the graphing. First we entered the numbers and created a graph.We made three graphs. They are temperature vs. the volume of gas, temperature vs. density, and mass vs. volume.
In the first graph, temperature vs. the volume of gas, shows that the volume of gas raises when the temperature is high. 


In the second graph temperature vs. density shows that the density before 4 degrees is increasing, when the temperature reaches 4 degrees, density reaches the highest point,1. Then it begins to drop as the temperature keeps on increasing.

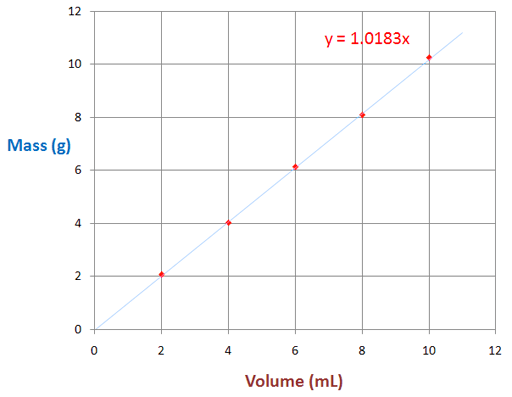
In the third graph mass vs. volume, the volume increases while the mass gets higher.

From the formula of the line, you get easily get the slope by looking at the constant before the x. Slope is equal to rise/run, or in this case, volume/mass. So to get density, find the reciprical of the slope.
Here's a helpful little video to show you how to make one.
No comments:
Post a Comment